This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
With 410 million injections worldwide, SUPARTZ FX is one of the most trusted hyaluronic acid treatments on the market.37 It has an unmatched history of performance—proven safe, effective, and affordable, with no pseudoseptic adverse events, based on post-market integrated analysis in more than 30 years of use.‡41
‡Does not include current prescribing experience.
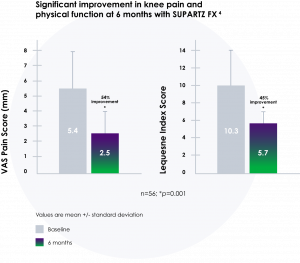
SUPARTZ FX has demonstrated effectiveness for up to 6 months22
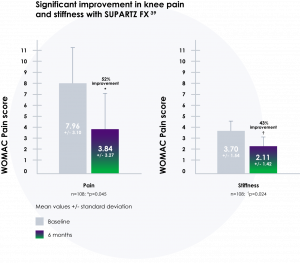
SUPARTZ FX significantly relieved osteoarthritis pain, a long-lasting benefit that lasted at least 18 weeks in a multicenter, prospective, randomized, double-blind, placebo-controlled study of 240 patients with knee osteoarthritis.39
- Delivers long-lasting benefits22
- Provides significant pain relief versus placebo (phosphate-buffered saline) in
knee osteoarthritis38
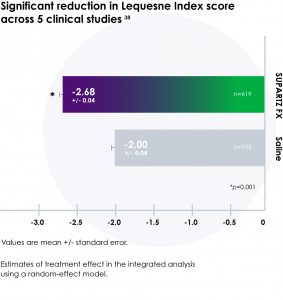 *The clinical relevance of this information has not been determined.
*The clinical relevance of this information has not been determined.
- Demonstrated significant improvements in multiple clinical parameters39
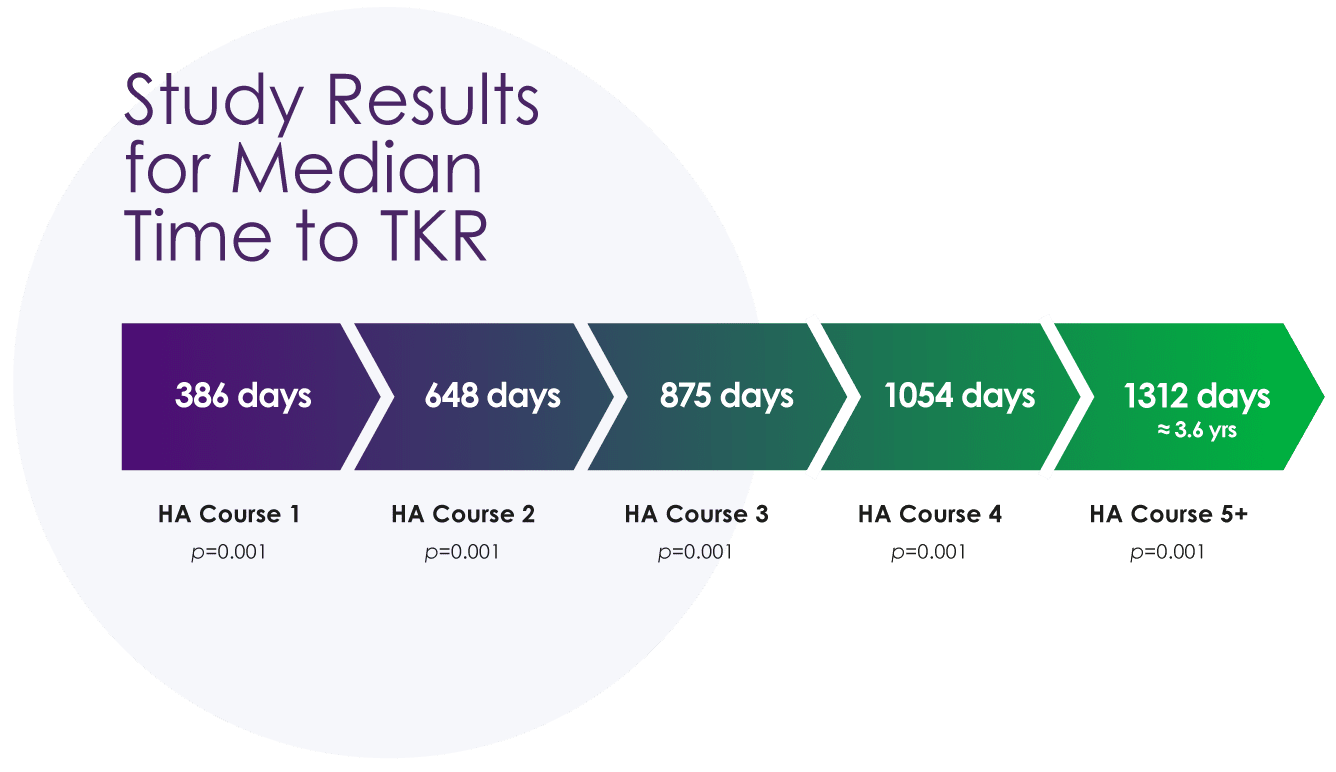
Median time to total knee replacement (TKR) is directly correlated with the number of courses of hyaluronic acid therapy12
In a retrospective analysis of a healthcare claims database of about 79 million patients who had undergone TKR, prior treatment with hyaluronic acid including SUPARTZ FX delayed the time to total knee replacement by up to 3.6 years.‡12
‡TKR delay with ≥5 HA treatment courses